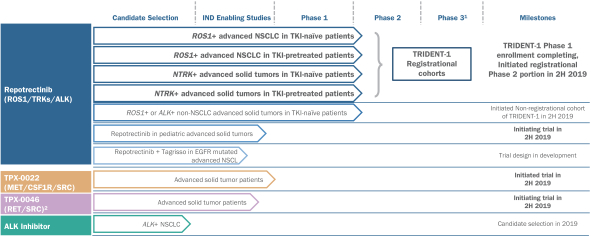
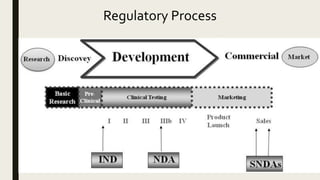
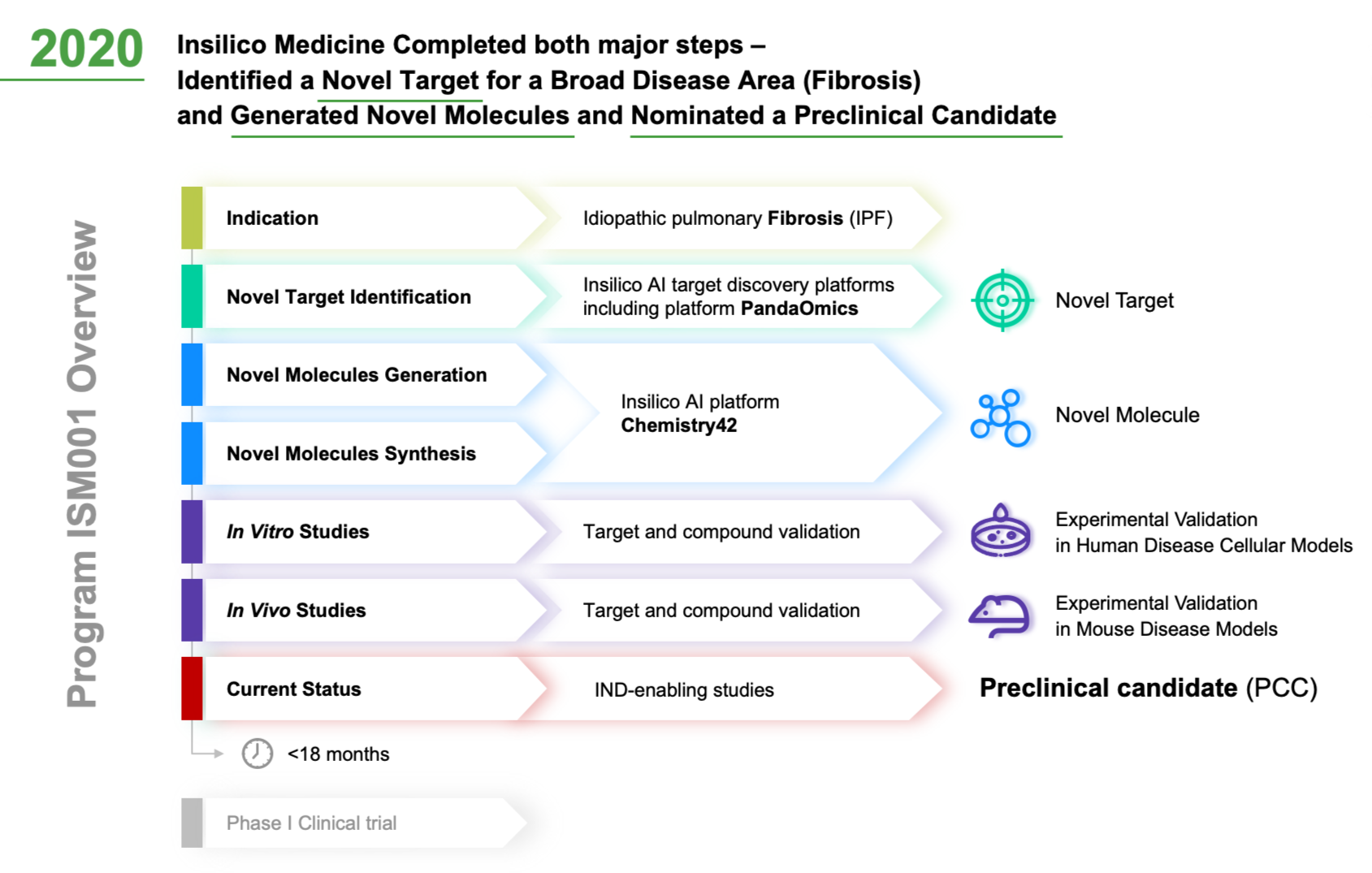
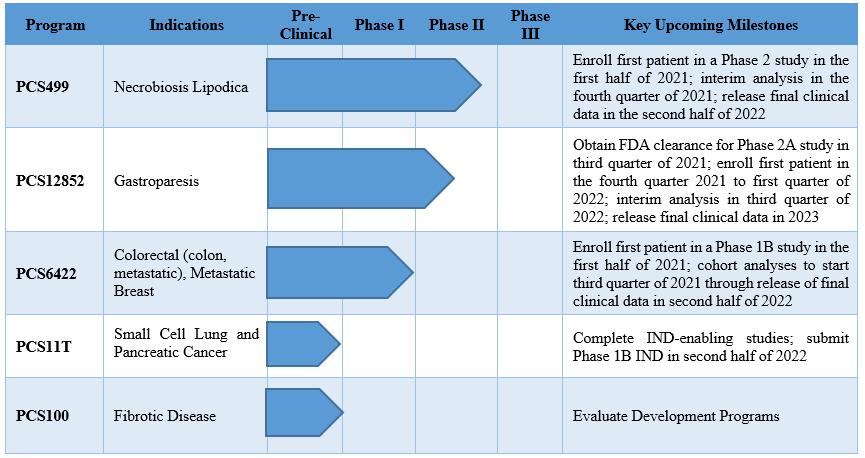
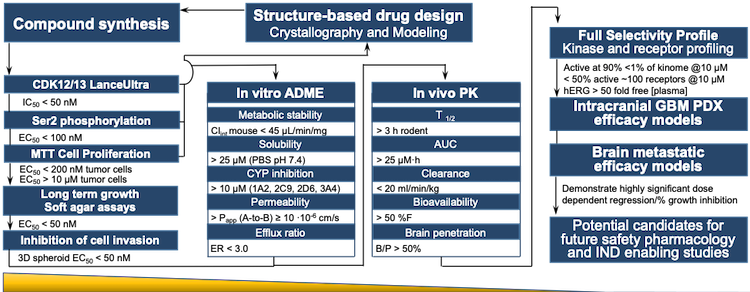
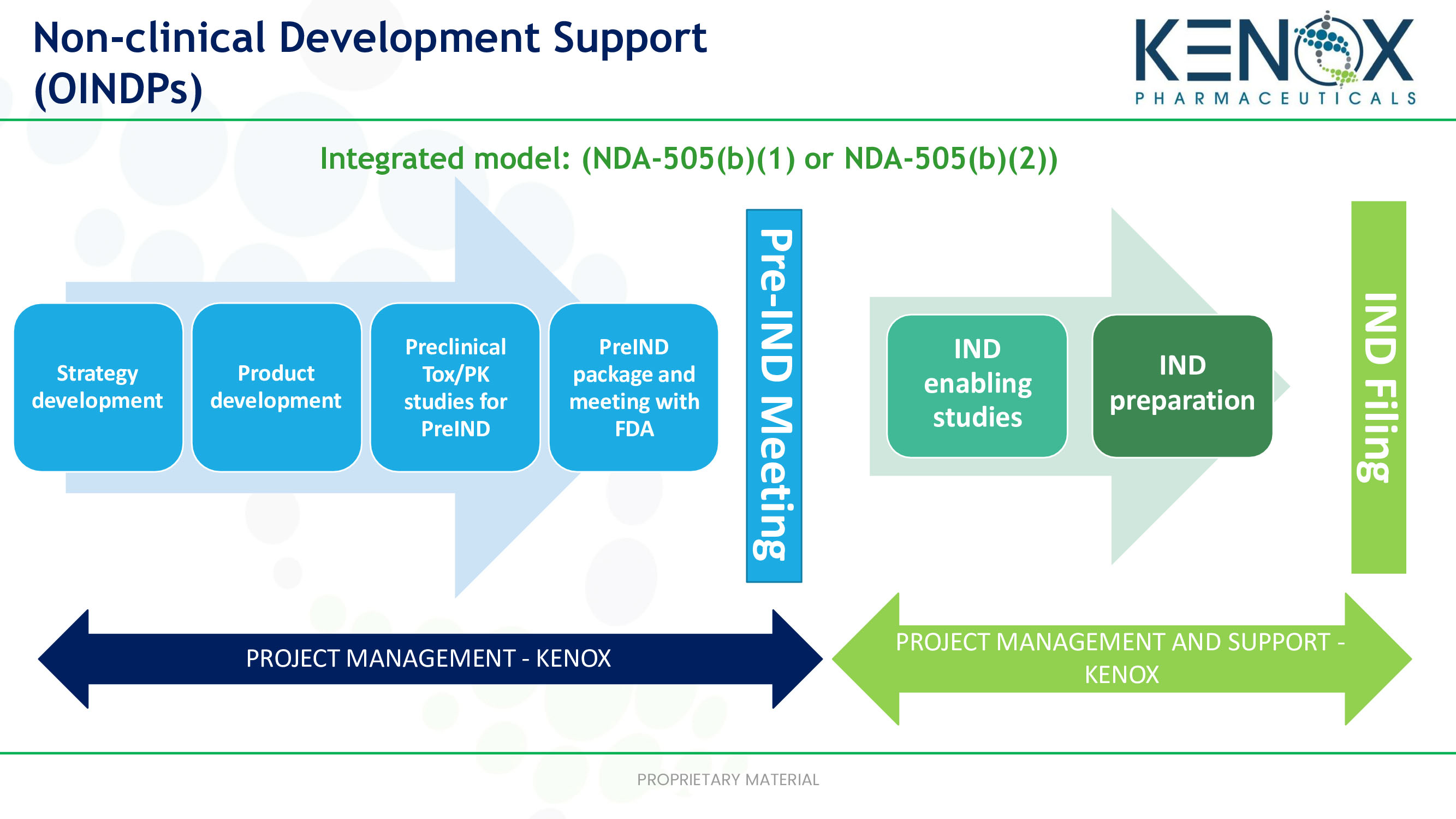
Verve Therapeutics on X: "Today we are excited to announce anticipated 2022 milestones including timing of first HeFH patient treated with VERVE-101 & initiation of IND-enabling studies for our ANGPTL3 program. Read
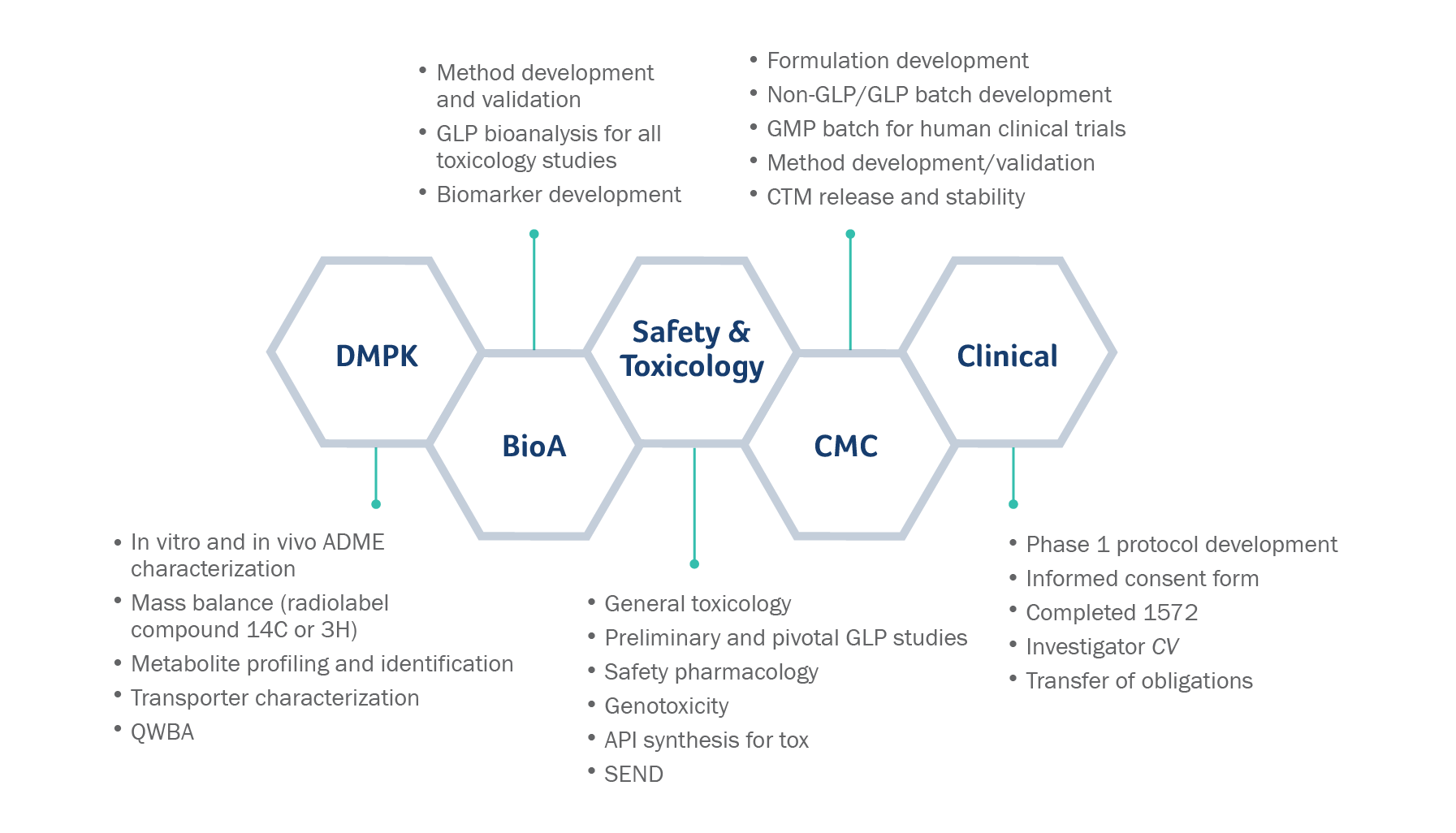
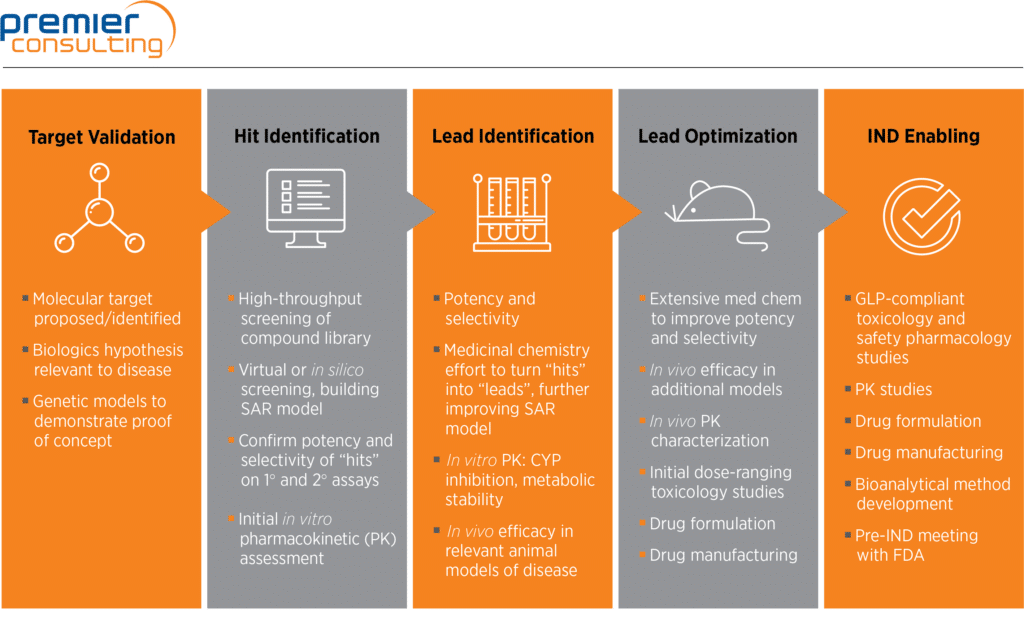
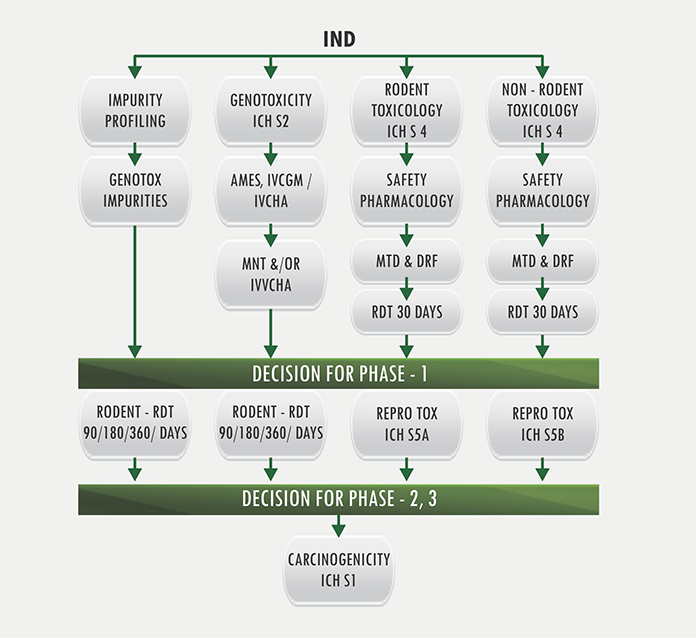
Preclinical pharmacology in IND-enabling studies and clinical pharmacology in clinical protocol development